The US Food and Drug Administration on Monday approved the use of the experimental drug aducanumab for early phases of Alzheimer"s disease — despite an FDA advisory committee concluding last year that there is not enough evidence to support the effectiveness of the treatment.
The drug was developed for patients with mild cognitive impairment, not severe dementia, and intended to slow the progression of Alzheimer"s disease — not just ease symptoms.
The FDA has not approved a novel therapy for Alzheimer"s disease since 2003.
"We have to really temper expectations"
The FDA approved aducanumab, also known as Aduhelm, using its "accelerated approval" program, which allows for the earlier approval of a drug for a serious or life-threatening illness even though more study into the drug"s benefits may be needed.
"There has been considerable public debate on whether Aduhelm should be approved. As is often the case when it comes to interpreting scientific data, the expert community has offered differing perspectives," Dr. Patrizia Cavazzoni, director of the FDA"s Center for Drug Evaluation and Research, said in Monday"s announcement.
"At the end of the day, we followed our usual course of action when making regulatory decisions in situations where the data are not straightforward," she said, noting that the FDA ultimately decided to use accelerated approval and "concluded that the benefits of Aduhelm for patients with Alzheimer"s disease outweighed the risks of the therapy."
Under accelerated approval, the drug aducanumab will still be studied. With this program, drug companies are required to conduct "post-approval studies" known as Phase 4 confirmatory trials to verify that treatments have clinical benefits. If the confirmatory trial does not verify the drug"s benefit, then the FDA could remove the drug from the market.
"FDA will continue to monitor Aduhelm as it reaches the market and ultimately the patient"s bedside," Cavazzoni said.
In November, the FDA"s Peripheral and Central Nervous System Drugs Advisory Committee was asked to vote on several questions about the evidence of the drug"s effectiveness. In response to a question about whether it was reasonable to consider data from one positive study as the primary evidence of aducanumab"s effectiveness for the treatment of early Alzheimer"s disease, none of the committee members voted yes — 10 voted no and one was uncertain.
The committee"s opinions were then left with the FDA as the agency mulled whether to approve the drug or pump the brakes.
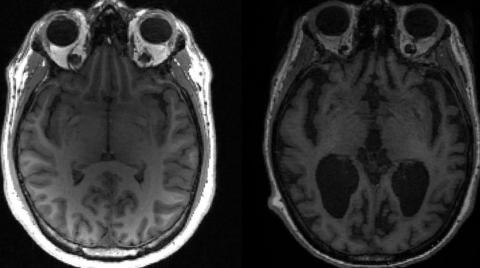
"In all studies in which it was evaluated, however, Aduhelm consistently and very convincingly reduced the level of amyloid plaques in the brain in a dose- and time-dependent fashion," Cavazzoni said on Monday. "It is expected that the reduction in amyloid plaque will result in a reduction in clinical decline."
The pharmaceutical company Biogen and its Japanese partner Eisai developed aducanumab, administered through intravenous infusion to treat early Alzheimer"s disease. The drug was developed for patients with mild cognitive impairment, not severe dementia. — CNN