To my fellow travellers, I’m sure the package I’m carrying looks like a lunchbox. Circular, and dark blue, with a Tupperware-style lid, it is precisely the kind of vessel you’d transport a soup or salad in. I’ve even sealed it inside a freezer bag, to contain any leaks. Or smells.
I walk slowly and with care across Westminster Bridge, because any trip could prove disastrous. As I enter St Thomas’ Hospital and head for the infection department on the fifth floor, I realise the object I’m carrying is still warm, and, despite my preparations, I’m sure I can detect a faint whiff of something ripe, like camembert.
It is, in a word, a turd. Freshly laid, and brimming with bacteria, the doctors I’m delivering it to believe such faeces could be the future of medicine. I’ve carried mine across London to be made into capsules – that someone else will ultimately eat.
If the thought of swallowing the bacteria from another person’s poo fills you with horror, consider Carol Goble. A string of infections with Clostridioides difficile (C. diff) – a gut bacterium that can cause severe diarrhoea and stomach cramps – had left her afraid to leave the house, or even eat. Antibiotics didn’t help, and by the time she was offered capsules of another person’s poo bacteria, she’d lost three stone in weight. For her, a faecal microbiota transplant (FMT), as it is officially known, was transformative. “It has completely changed my life and I feel like I’m back to myself again – it is amazing,” she said.
The human gut contains trillions of bacteria, many of which play important roles in keeping us healthy, but occasionally a few microbiological “bad apples” can throw the whole system out of whack. Increasingly, though, doctors are realising that restoring a healthy balance of bacteria could help to treat a range of different ailments, from brain disorders to metabolic disease, to arthritis. It could even improve cancer patients’ responses to treatment. And it all begins with a poo like mine.
The idea of using faeces to treat disease isn’t entirely new. Approximately 1,700 years ago, a Chinese doctor called Ge Hong urged patients suffering from severe diarrhoea to consume “yellow soup” to cure their illness, a practice that apparently continued for centuries.
More recent attempts at FMT have largely involved introducing it from the other end of the gastrointestinal tract – most commonly via a long thin instrument that deposits the preparation in the colon. For now, the only licensed application is the treatment of recurrent C. diff infections – although clinical trials exploring other disease areas have exploded in recent years.
C. diff bacteria usually live harmlessly in the colon alongside other species, but sometimes they start to take over – most commonly after a course of antibiotics – and produce a toxin that makes their hosts unwell. Such infections can prove fatal; in the US, C. diff infection is associated with 15,000-30,000 deaths each year. Standard treatment involves antibiotics, but about 20% of patients develop repeated infections, like Goble.
Since 2014, the UK’s National Institute of Health and Care Excellence (Nice) has recommended FMT as an alternative for patients who have experienced at least two previous infections. The success rate is impressive, with about 85% of people cured after a single transfer. In November, the US Food and Drug Administration also approved its first faecal microbiota product – a liquefied preparation of stool, administered via an enema into patients’ intestinal tracts.
Although delivering bacteria via the back passage is effective, it isn’t particularly comfortable or convenient for patients.
Enter capsules – or “crapsules” as they’re known in the trade. Compared with traditional FMT, “they are a gamechanger in terms of making FMT far more accessible, easier and cheaper,” says Prof Simon Carding at the Quadram Institute in Norwich, who plans to launch his own trial of FMT to treat myalgic encephalomyelitis (ME) later this year.
By the time I arrive at the St Thomas’ lab carrying my precious cargo, a battery of clinical tests has already pronounced one of my previous poos to be free from parasites, Sars-CoV-2, antibiotic-resistant organisms and an array of other bugs capable of causing gastrointestinal illness. I’ve also been declared generally fit and healthy, and free of various blood-borne viruses, including HIV and hepatitis.
Such rigorous screening is essential, and one reason why DIY faecal transplants are to be avoided. “You wouldn’t want to just take any person’s stool sample and turn it into a capsule and swallow it without checking to see that they don’t have any diseases and infections that they can transmit,” says Dr Simon Goldenberg, consultant microbiologist and infection control doctor at Guy’s and St Thomas’ NHS Foundation Trust.
Désirée Prossomariti, a research biomedical scientist, takes my sample and opens it inside a biosafety cabinet. Watching her scoop my poop into a series of plastic containers feels uncomfortable: it’s not often we hand over something so intimate, nor so laden with cultural taboos, to a fellow human.
Prossomariti reassures me that she views faeces as “just another type of specimen”. “A lot of people get grossed out by it, but it is not much worse than blood. Besides,” she adds, “in my personal opinion, it is not the most disgusting thing you can work with.”
“What is?” I ask.
“Sputum,” she says. “That’s awful.”
The samples she is preparing will undergo further tests, to ensure I haven’t picked up any additional bugs since my last screening. The rest of my poo is weighed, and two-thirds of it – 100g – is scooped into a sterile flip-top sports bottle, along with 200ml of saline, and shaken into a slurry. The liquefied poo is filtered then centrifuged to remove any undigested food; this suspension is spun again at a higher speed to remove the saline and concentrate the bacteria into a soft pellet. Finally, Prossomariti adds a sugar solution, which will protect against ice-crystal formation during the next step, and tips the silky brown fluid into a petri dish. She puts this into a freeze dryer overnight, to remove any remaining water.
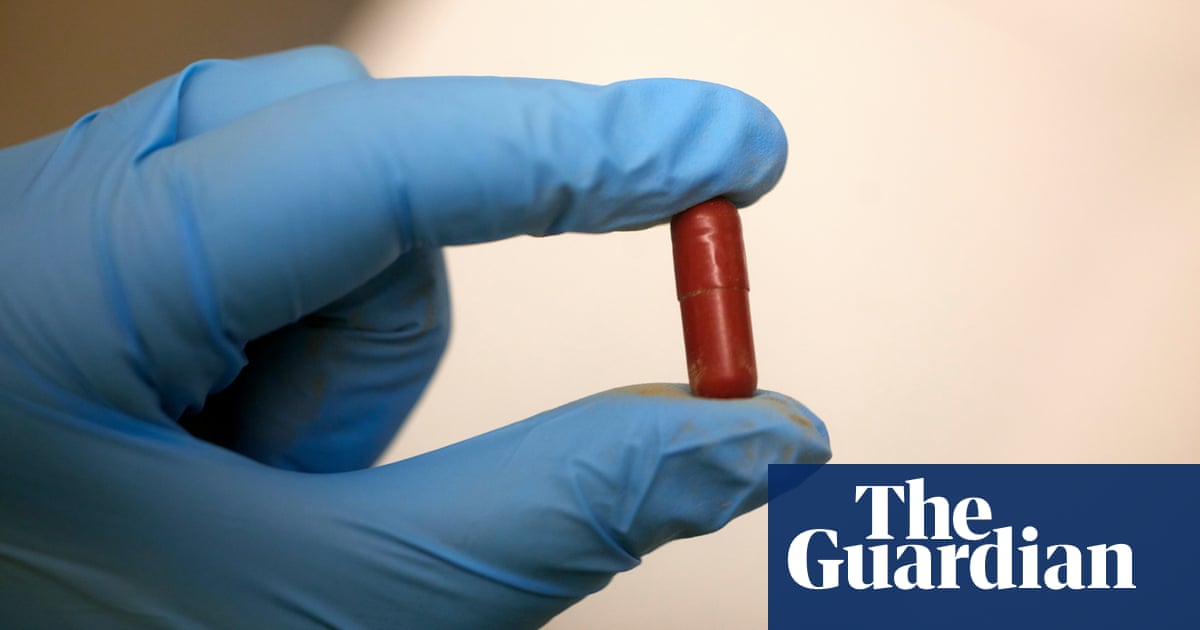
The bacterial cake that emerges is exquisite: pale golden brown, and etched with fine lines that give it the appearance of cross-sections of wood, or a dusty window that Jack Frost has gone to work on. Prossomariti takes this wondrous material and crushes it into a dusty powder, which she scoops and packs into five large red capsules.
Holding one in your hand, you’d never guess what it contained. Weighty, yet odourless, they are designed not to spill their contents until they’ve passed through the stomach and entered the small intestine. In truth, they look unimpressive: like any other drug you might receive in hospital.
But who would swallow such a medicine?
Helen, 45, is one of about 40 patients enrolled on a trial at St Thomas’ Hospital to test whether FMT could help eradicate antibiotic-resistant bacteria from people’s bowels. Such organisms present a growing and serious threat to global health, rendering even relatively simple infections potentially deadly.
Helen experienced unpleasant bloating after catching typhoid in Ghana and being treated with antibiotics for it. Further tests revealed antibiotic-resistant bacteria living in her bowels. When she was invited to participate in the trial, she didn’t hesitate.
“Ultimately, I thought ‘I’ve got nothing to lose, and if this can help, not just personally, but with the research, then why not?’” she says. “We put our bodies through much worse things than this – even alcohol or smoking – so I was sure I’d be able to cope.”
Though Helen doesn’t know whether she received bacterial capsules or placebo ones, her symptoms have cleared up: “I’m hoping they gave me the real stuff, and that they’re doing their job.”
Antibiotic-resistant bacteria are just the start. Other labs are exploring whether FMT could be used to treat arthritis, type 1 diabetes, or even help people with neurological disorders, such as autism and Parkinson’s disease.
Modifying someone’s gut bacteria to treat gastrointestinal disorders makes intuitive sense, but it is a greater leap to see how it could be beneficial for some of these other disorders. Considering their wider relationship with the immune system is a good starting point, says Prof Tariq Iqbal, a consultant gastroenterologist and co-founder of University of Birmingham’s Microbiome Treatment Centre. “The gut is our biggest interface between the environment and the immune system, and is key to programming it. Certainly, in a condition like inflammatory arthritis, you could down-regulate an overactive immune response by treating an imbalance in the gut microbiome.”
When it comes to brain disorders, such as Parkinson’s, the mechanism may be more complicated. However, one theory is that an overgrowth of harmful gut bacteria could damage the gut lining, allowing substances that would normally be excluded from the blood to filter through.
Another possibility is that certain gut bacteria produce substances that can get into the blood, and damage tissues further afield.
FMT capsules aren’t the only option being explored. At Microbiotica, a biotech company based near Cambridge, researchers are trying to identify populations of beneficial gut microbes that could be cultured in fermentation tanks, and then packaged into oral capsules, without ever having seen a poo.
“The problem with [existing products] is they are donor-derived, which means you are going to have a variable product,” says Tim Sharpington, Microbiotica’s CEO. “Certain donors produce fantastic response rates, and others less so. This leads us to the conclusion that there are certain bugs or groups of bugs in there which are good, but there are also certain bugs which are bad.”
Microbiotica’s scientists are trying to identify which of the thousands of microbial species in a typical gut are responsible for the beneficial effects of FMT. The hope is that these could be individually grown in fermentation tanks, and packaged together into capsules to produce more consistent therapeutic effects. And they are setting their sights on one of the most feared diseases of all.
Cancer immunotherapy can be extraordinarily effective, but it only works in a subset of patients – typically about 40% in the case of patients with melanoma skin cancer. Identifying them is still something of a guessing game but, increasingly, studies are hinting that there may be differences in microbiomes of immunotherapy responders and non-responders.
This research took a compelling twist in 2021, when two separate research groups, one in the US, the other in Israel, announced that they had taken stool material from advanced melanoma patients who had responded well to immunotherapy drugs called checkpoint inhibitors, and transferred it into non-responders, prompting some of these patients to show improved clinical responses.
Microbiotica has since analysed this and other data to try to identify which bacteria were responsible for the effect. All of the bacteria are commensals, meaning they live on our body surfaces without causing us harm, and may perform useful functions, such as protecting us against pathogens or training our immune cells to function properly. Sharpington won’t divulge the names of the bacteria, not least because four of the organisms are “unknown to man, outside our company”.
He believes the bacteria are interacting with immune cells called dendritic cells residing in the gut lining, which can tweak the tone of the wider immune system. Quite possibly, different subgroups of bacteria have different therapeutic effects: those that appear beneficial in inflammatory diseases such as arthritis or ulcerative colitis, seem to dampen certain immune responses, whereas those associated with people’s response to cancer immunotherapy have stimulatory effects, Sharpington says. The company plans to launch its own trial of the bacteria it has identified in melanoma patients later this year.
Identifying and growing specific species of bacteria for transplantation won’t necessarily be straightforward, warns Iqbal: “The gut microbiome consists of trillions of microbes. Just choosing 15 or so different components, and hoping they’re going to engraft into that ecological niche, is quite a difficult thing to do.” Even so, he agrees this approach is probably the future of FMT. The adage “one man’s trash is another man’s treasure” never rang truer.
In the future, people might consider banking their own stool in youth, just as they do their eggs or their babies’ cord blood, in case they need to repopulate their gut with healthy bacteria in later life.
There’s still a way to go before faecal transplants, in whatever form, make it into mainstream medicine – although recent Nice and FDA recommendations for C. diff show that it is possible. Getting over the “yuk” factor remains a significant barrier: according to Goldenberg, less than a third of patients with antimicrobial-resistant infections that he approached were willing to enrol on his oral capsule trial – although that could change if such treatments proved effective in large-scale clinical trials.
If they do, faecal transplants could provide an affordable solution to some of mankind’s most pressing challenges – from diseases of ageing to antimicrobial resistance. And who could pooh-pooh that?












