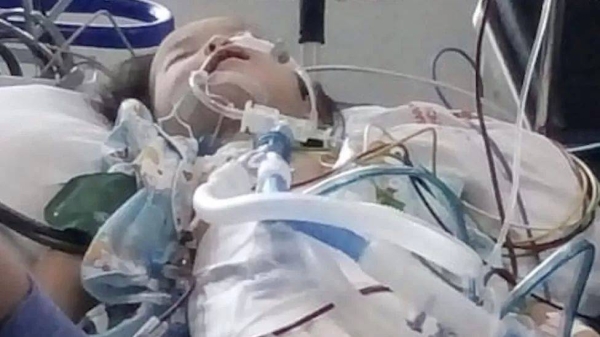
The last time three-year-old Anirudh was captured on video, he lay listlessly on a hospital bed with wires attached to his hands.
His mother is seen desperately trying to feed him, talking about the games he can play once he recovers.
Three days later, on 10 January 2020, the child died in the hospital.
For a fortnight before his death, he had suffered from diarrhea and vomiting and was unable to urinate. His parents say these symptoms began after they gave him a cough syrup — bought over the counter from a local chemist in Ramnagar, a small town in the northern Indian region of Jammu, where they live — to tackle a fever and chest infection.
Doctors at the hospital said Anirudh"s kidneys were damaged, and that levels of creatinine — a waste product normally filtered out by the kidneys — were very high in his body.
His parents allege his death was caused by the cough syrup — local drug control officials say that tests showed it contained high amounts of diethylene glycol, a toxic compound that could cause kidney failure and death if ingested.
"The child suffered a lot," his mother Veena Kumari told the BBC. "He had difficulty consuming food, couldn"t open his eyes and his face and body were swollen."
Between December 2019 and January 2020, at least 12 children — all under the age of five — died in Ramnagar, allegedly after drinking the cough syrup. Activists have said the number of deaths could be higher.
Parshottam Goyal, the owner of Digital Vision, which made the cough syrup, denies that their medicine was responsible for the deaths.
"Why would we kill someone"s children? There are children in our houses too. We manufacture medicines, not poison," he told the BBC.
After child deaths in The Gambia and Uzbekistan were linked to Indian-made cough syrups, the Ramnagar tragedy came back into focus, giving the parents a chance to demand justice.
Many of the parents say that their children experienced symptoms similar to those shown by children in The Gambia and Uzbekistan before they died.
India is the world"s largest exporter of generic drugs, meeting much of the medical needs of developing countries. But the deaths in The Gambia and Uzbekistan made international headlines, raising questions about quality standards in India"s pharmaceutical industry.
Indian health activists say they have long tried to raise the alarm about lax manufacturing practices and regulatory oversight around the booming industry.
In their book, The Truth Pill, health expert Dinesh Thakur and advocate Prashant Reddy write that India"s first recorded case of diethylene glycol poisoning was in 1972, when 15 children died in the southern state of Tamil Nadu.
Since then, there have been "mass poisoning events" in several Indian states, they say, adding that the death tolls may be much higher as diethylene glycol poisoning is hard to diagnose.
The authors say companies usually don"t "test either the raw materials or the final formulation before shipping it to the market".
However, Indian regulators say that the deaths in Ramnagar are one-off instances.
"Based on aberration and a few batches, you cannot say the entire pharmaceutical industry of India is not doing well," says Udaya Bhaskar, director general of the Pharmaceuticals Export Promotion Council of India.
Indian regulators have said that the four cough syrups linked to child deaths in The Gambia complied with specifications when tested at home — which the World Health Organization has contested. It did, however, cancel the manufacturing license of the firm whose products allegedly led to fatalities in Uzbekistan.
It has also made it compulsory for cough syrup makers to get samples tested before exporting their products.
In the Ramnagar case, it took two years for police to file charges against five people, including the chemist who sold the syrup and three officials of Digital Vision — the case is being heard in a local court. A top district police official did not respond to the BBC"s interview request.
Digital Vision"s manufacturing unit in Ramnagar was closed for six months in 2020, but was reopened after a court allowed it to operate.
The parents say that they want action to be taken against those responsible for the deaths of their children.
"We want justice. We want the killers to be punished," says Murfa Biwi, whose three-year-old son, Irfan, died 10 days after consuming the syrup.
"The victims died for no reason. The manufacturer and drug control officers failed to perform their duty," alleges Sukesh Khajuria, an activist in Jammu, who is helping the parents in their fight.
He adds that if government officials had done their jobs, "there would have been no [deaths in] Gambia".
But drug control officials insist they worked hard to deliver justice.
The samples of the cough syrup collected in Ramnagar and sent for testing to a lab in Chandigarh had "more than 34% diethylene glycol," Jammu and Kashmir drug controller Lotika Khajuria told the BBC. The samples" findings were corroborated by another Kolkata-based lab, according to Ms Khajuria.
A team of experts led by paediatrician Bhavneet Bharati who investigated the deaths reached the same conclusion.
"The toxins primarily failed their kidneys; in some, multiple organs were affected — brain, liver, lungs. Some of them had to be put on a ventilator. Some died while others were left with major disabilities," Ms Bharati said.
One of the survivors is Pawan Kumar, who was 15 months old when he consumed the same cough syrup. He was in a hospital for three months before returning home, and is still being treated.
"His eyesight has diminished, listening capacity has significantly reduced and he has high blood pressure," says Shambhu Ram, his father.
The families want the government to financially assist with their children"s treatment. — BBC