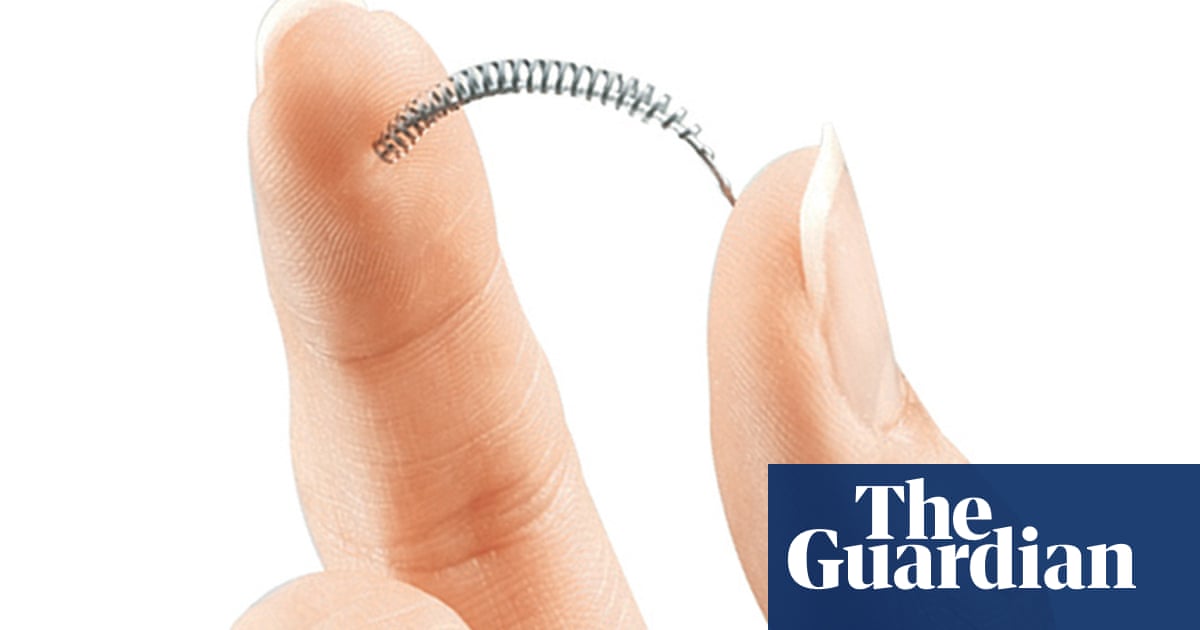
Two hundred women in the UK who say they were left in pain after having a permanent contraception device fitted have permission to launch a group legal action against its manufacturer, it has been reported.
The device, known as Essure, is a soft, flexible device placed into the patient’s fallopian tubes. Over three months a barrier forms around the inserts, which is intended to block the fallopian tubes and permanently prevent pregnancy.
But there have been reports women experienced changes in menstrual bleeding, unintended pregnancy, chronic pain, perforation and migration of the device, allergic reactions and immune-type reactions after receiving the implant.
The manufacturer, the pharmaceutical company Bayer, is facing legal action around the world in relation to the device.
Lawyers in England began legal action in 2020 and now have permission to bring a group claim on behalf of 200 women, the BBC reported. Bayer told the broadcaster it would vigorously defend the claims.
A company official told the BBC: “Bayer’s highest priority is the safety profile and effectiveness of our products and we have great sympathy for anyone who has experienced health problems while using any of our products, regardless of cause.
“The company stands by the safety profile and efficacy of Essure and will continue to defend itself from these claims vigorously.”
The company and other researchers had completed 10 clinical trials and more than 70 real-world observational studies, it said.
“While all birth control products and procedures have risks, the totality of scientific evidence on Essure demonstrates that the benefit-risk profile is positive,” it said.
A 2017 study published in the British Medical Journal found women implanted with Essure had a significantly heightened risk of needing further surgery due to complications than those using the more traditional laparoscopic procedure.
The study found use of the device was associated with a more than 10-fold higher risk of additional surgery, equivalent to about 21 additional re-operations per 1,000 patients. The authors warned the device was a “serious safety concern”.
Bayer has already paid out more than $1.6bn (£1.3bn) in the US to resolve claims from nearly 39,000 women, but admits no wrongdoing or liability.












