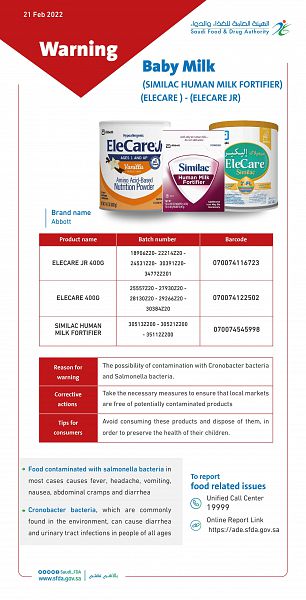
Riyadh, February 21, 2022, SPA -- The Saudi Food and Drug Authority (SFDA) has warned against three baby milk powder products produced by one of the facilities of Abbot” company in the United States of America, which are (ELECARE JR), (ELECARE) and (SIMILAC HUMAN MILK FORTIFIER), due to the possibility of contamination with Cronobacter bacteria and Salmonella bacteria.
SFDA published, on its official website, an explanatory table of the affected products, including the names of the products, batch numbers and the product identification number "barcode".
SFDA noted that other batches produced by the company are not included in this warning. SFDA indicated that it is taking the necessary measures in coordination with the importing company in the Kingdom and the competent authorities to follow up not to enter the suspected contaminated products to the Kingdom's markets and to withdraw the offered products from the local markets.
SFDA clarified that food contaminated with salmonella bacteria in most cases causes fever, headache, vomiting, nausea, abdominal cramps and diarrhea, while Cronobacter bacteria, which are commonly found in the environment, can cause diarrhea and urinary tract infections in people of all ages, so it is important to follow the instructions for the preparation, handling and storage suitable for
preparing baby milk.
SFDA advised consumers to avoid consuming these products and dispose of them, in order to preserve the health of their children, and the affected milk products can be confirmed through the Tammni application or visit SFDA website via the following link: www.sfda.gov.sa.
--SPA
15:43 LOCAL TIME 12:43 GMT
0015
www.spa.gov.sa/w1694402












