RIYADH — The Saudi Food and Drug Authority (SFDA) has approved Casgevy, the first gene therapy for sickle cell anemia and thalassemia diseases in patients 12 years of age and older.
It is a cell-based gene therapy, utilizing CRISPR/Cas9, a type of genome editing technology. Patients’ hematopoietic stem cells are modified by genome editing using CRISPR/Cas9 technology. CRISPR/Cas9 can be directed to cut DNA in targeted areas, enabling the ability to accurately edit (remove, add, or replace) DNA where it was cut.
The authority said that the effectiveness, safety and quality of the treatment was evaluated, after meeting the necessary standards, as the treatment works by genetic editing of the genetic mutation in the affected gene, so that the body can produce hemoglobin properly. This is through extracting stem cells from the patient’s bone marrow and genetically editing them in the laboratory, and then it is re-implanted into the patient’s body to give a long-term effect.
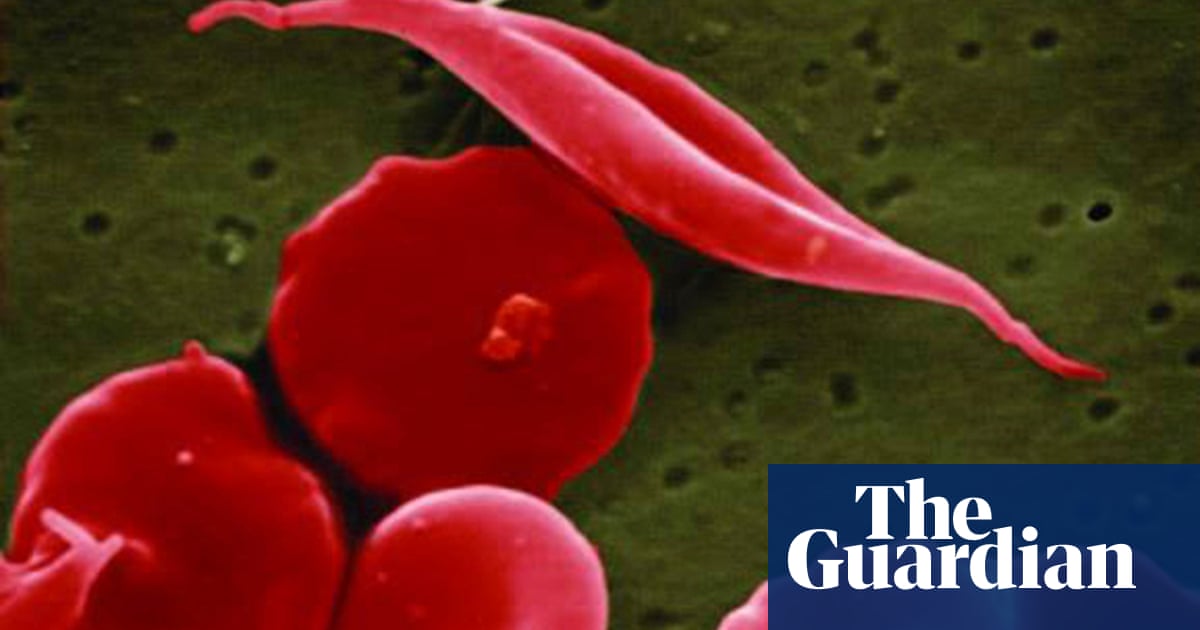
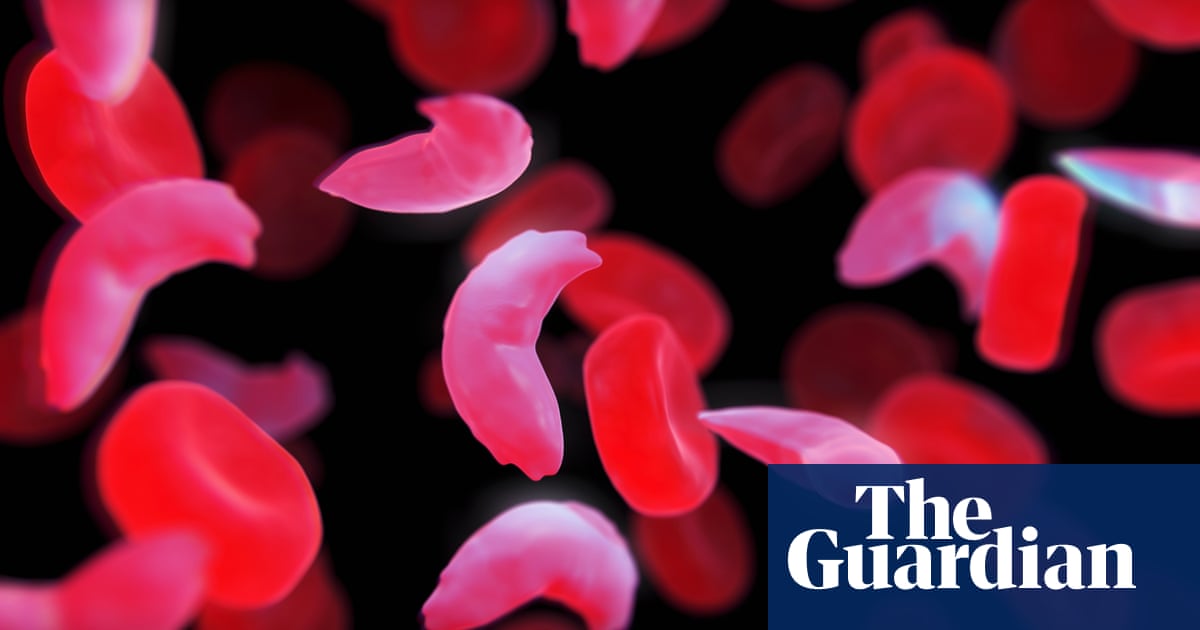
Sickle cell anemia is a hereditary blood disorder that affects hemoglobin, the protein that carries oxygen through the body. It occurs as a result of mutations in the genes responsible for the formation of hemoglobin. These mutations cause the shape of red blood cells to change from round to sickle, which makes it difficult for them to cross the veins, and causes severe pain and a lack of oxygen transfer to cells in the body. These are the symptoms associated with crises in people with sickle cell anemia, including severe pain and shortness of breath.
Thalassemia is a hereditary blood disease caused by genetic mutations that affect the production of hemoglobin, causing disruption of its natural production and a decrease in its levels in the blood. The accompanying symptoms include anemia and shortness of breath.