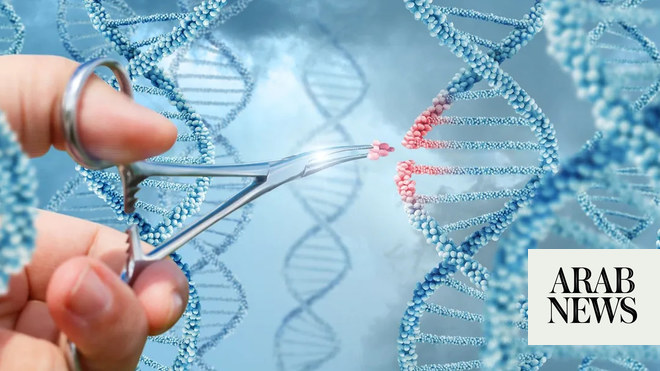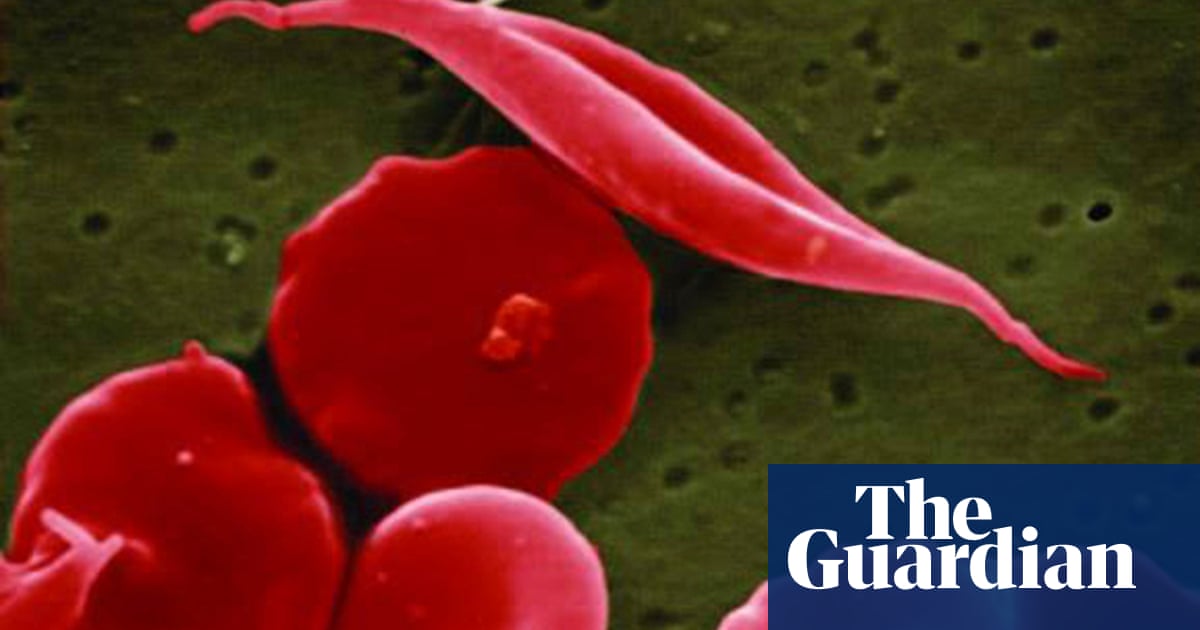
The US Food and Drug Administration has approved a pair of gene therapies for sickle cell disease, including the first treatment based on the breakthrough Crispr gene-editing technology, opening up two “transformative therapy” avenues for some patients.
The FDA approved Lyfgenia from Bluebird Bio, and a separate treatment called Casgevy by partners Vertex Pharmaceuticals and Crispr Therapeutics. Both therapies are made from the patients’ own blood stem cells and were approved for people aged 12 and older.
The Vertex/Crispr gene therapy uses the breakthrough gene-editing technology that won its inventors the Nobel prize in 2020. The therapy can be directed to cut DNA in targeted areas, enabling the ability to accurately remove, add or replace DNA where it was cut.
The modified blood stem cells are then transplanted back into the patient, where they attach and multiply within the bone marrow and increase the production of fetal hemoglobin, a type of hemoglobin that facilitates oxygen delivery.
Lyfgenia is a cell-based gene therapy that modifies a patient’s blood stem cells to produce a gene therapy-derived hemoglobin that functions similarly to a type of normal adult hemoglobin not affected by sickle cell disease.
Sickle cell disease is a painful, inherited blood disorder that can be debilitating and lead to premature death. It affects an estimated 100,000 people in the US, is most common in African Americans and, while less prevalent, also affects Hispanic Americans.
President Biden said in a statement that the approval of the treatments represented a “major breakthrough” for those living with the disease and noted that it disproportionally affected the two minority groups.
“My administration has worked tirelessly to close these health disparities and help deliver care for sickle cell disease patients and their families, and we will continue to do so,” he said, adding that his administration would continue “efforts to accelerate the development of cures for rare diseases and support the medical research and innovation”.
In sickle cell disease, the body makes flawed, sickle-shaped hemoglobin, impairing the ability of red blood cells to properly carry oxygen to the body’s tissues. The sickle cells tend to stick together and can block small blood vessels, causing intense pain. It also can lead to strokes and organ failure.
“Sickle cell disease is a rare, debilitating and life-threatening blood disorder with significant unmet need, and we are excited to advance the field especially for individuals whose lives have been severely disrupted by the disease by approving two cell-based gene therapies today,” said Nicole Verdun, director of the office of therapeutic products within the FDA’s Center for Biologics Evaluation and Research, said in a statement.
“Gene therapy holds the promise of delivering more targeted and effective treatments, especially for individuals with rare diseases where the current treatment options are limited,” Verdun added.
Makers of both the therapies have pitched them as one-time treatments, but data on how long their effect lasts is limited. The only longer-term treatment for sickle cell disease is a bone marrow transplant.
“I actually am very reticent to call them a cure. I prefer to call them a transformative therapy because patients will still have sickle cell disease on the other side of gene therapy,” said Sharl Azar, medical director of the Comprehensive Sickle Cell Disease Treatment Center at Massachusetts general hospital.
Both gene therapies can take several months and involve high-dose chemotherapy, but this has potential risks of infertility.
“Not everybody who undergoes chemotherapy will end up having infertility, but the majority of them will,” said Azar.
While the risk can be managed by fertility preservation methods such as freezing eggs and sperm banking, this is only covered by insurance for cancer patients who undergo chemotherapy and not those receiving gene therapy, said Azar.
He said the out-of-pocket expense on it can be as high as $40,000.
FDA staff in documents released ahead of an October meeting of a panel of independent experts on Vertex’s therapy had also flagged concerns of unintended genomic alterations from the treatment.
The company plans to assess potential long-term safety risks through a 15-year follow-up study after approval.